In an effort to reduce the population of non-native rainbow trout and benefit native Yellowstone cutthroat trout in the South Fork Snake River, Idaho Fish and Game has used boat electrofishing — a fish sampling technique that temporally stuns fish by applying electrical current to the waterway. This technique is used to capture and relocate rainbow trout from the South Fork to other waters where rainbow trout make fishing better without impacting native species.
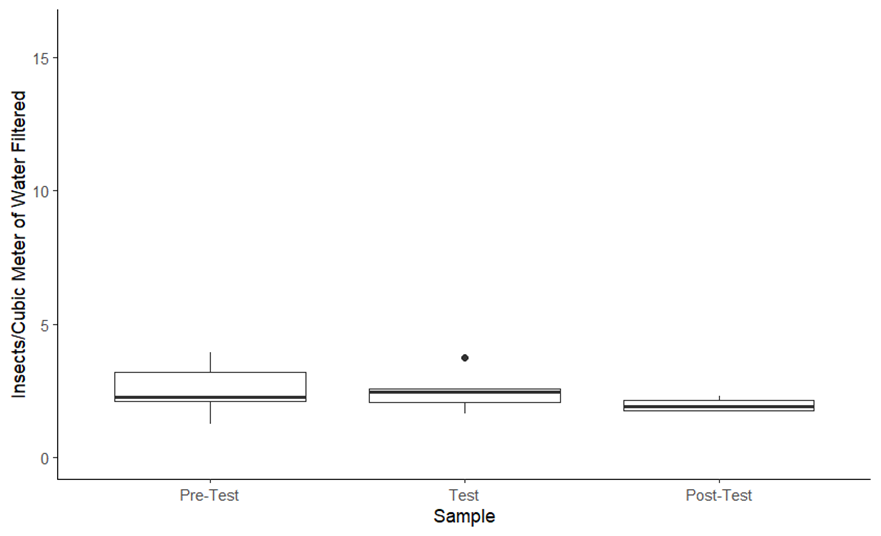
Electrofishing efforts have been met with some controversy from the angling public. A public meeting was held by Fish and Game in the spring of 2023 to address the concerns. During the meeting, a member of the public questioned whether these electrofishing efforts have had a negative impact on aquatic insect populations. While a few studies have researched impacts of backpack electrofishing on aquatic insects, we are not aware of published articles investigating impacts of boat-based electrofishing. Stable aquatic insect populations are vital to maintain a healthy fishery, so Idaho Fish and Game in collaboration with BYU-Idaho studied whether jetboat and raft electrofishing increased the rate in which aquatic insects are disturbed from the riverbed (drift rate) and insect mortality.